Today, we shared some big news! Medtronic announced its intent to separate our Diabetes business into a standalone, public company.
I wanted to personally reach out regarding the recent approval of our Simplera Sync™ sensor. While this should have been a moment of celebration, I understand many of you were disappointed by our communication.
Hear from Medtronic Champions on how they’ve embraced an active life with their tubed insulin pumps.
If you are on a current Medtronic pump, learn how to upgrade to the MiniMed 780G system.
Just like his favorite superhero, Max gets his superpowers from his life-saving device. Hear his story.
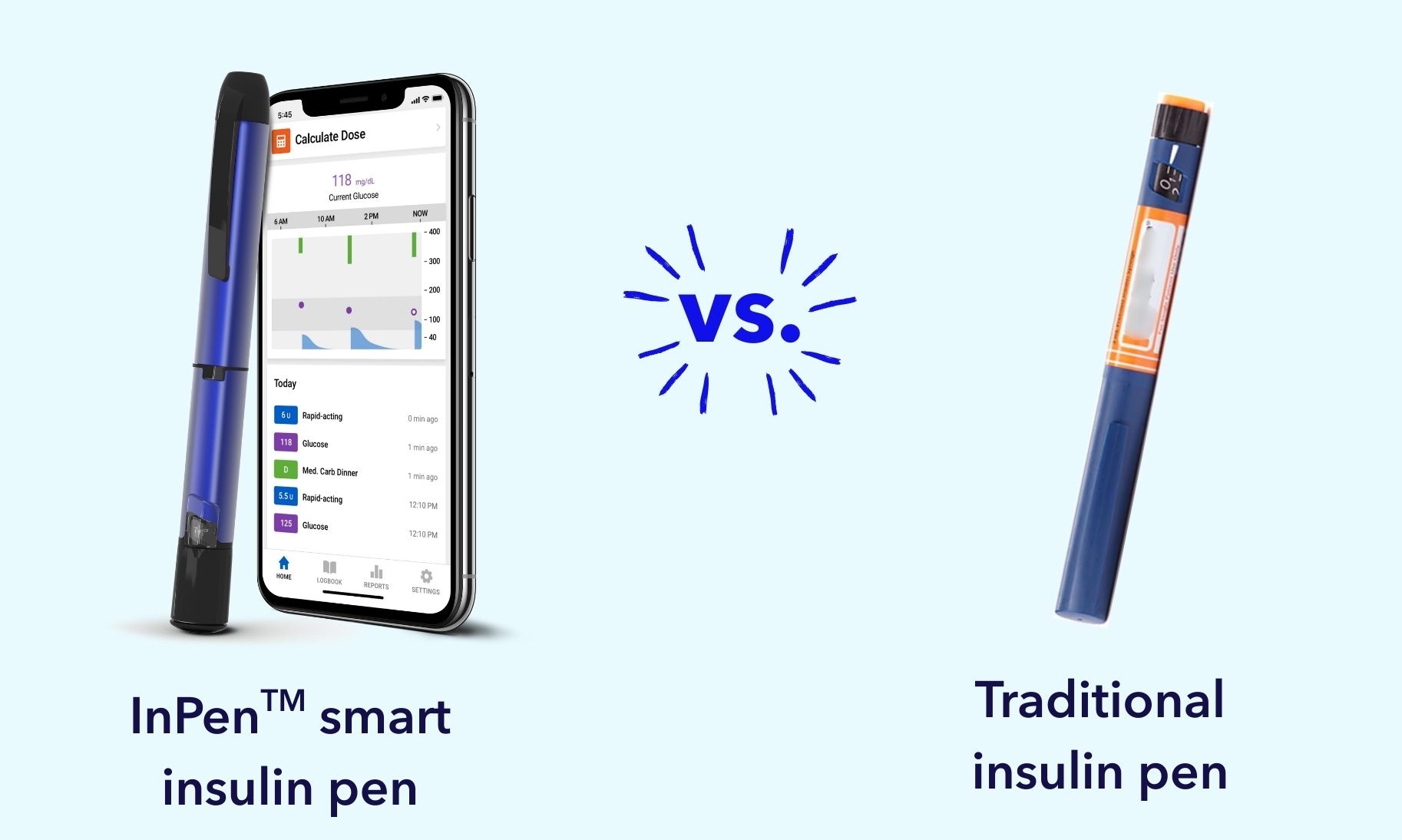
Medtronic access programs that may help reduce the cost of insulin pumps, CGMs, smart insulin pens, and ongoing supplies.
Learn how to update your insulin pump settings for Daylight Saving Time.