Medicare for diabetes management
Medicare and Medicare Advantage customers can access Medtronic solutions.
Medicare and Medicare Advantage customers start here

Whether you’re new to Medicare coverage or new to Medtronic diabetes products through Medicare, you may have a lot of questions — most importantly around coverage for your supplies. We are here to help!
Medtronic offers integrated solutions to meet your lifestyle so you can think less about diabetes and more on what matters. Learn more about our solutions or visit the frequently asked questions.
Existing customers who have newly qualified for Medicare can learn about next steps.
Medicare FAQ
Explore our integrated solutions
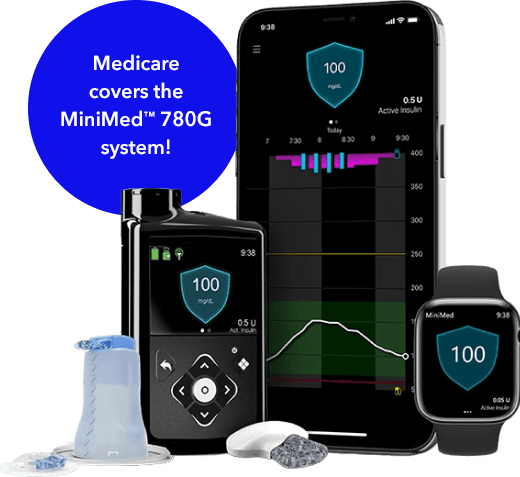
Automated Insulin Delivery System
For people who prefer an insulin pump
Smart devices sold separately.
If you have type 1 diabetes, an insulin pump system could help prevent highs and lows with real-time insulin delivery adjustments 24/7— meaning less work for you!
With an insulin pump system, you get:
- Easier mealtime dosing with Meal Detection™ technology*
- Automatic adjustments and corrections every 5 minutes§
- 96% fewer injections than daily insulin injections**
Learn more
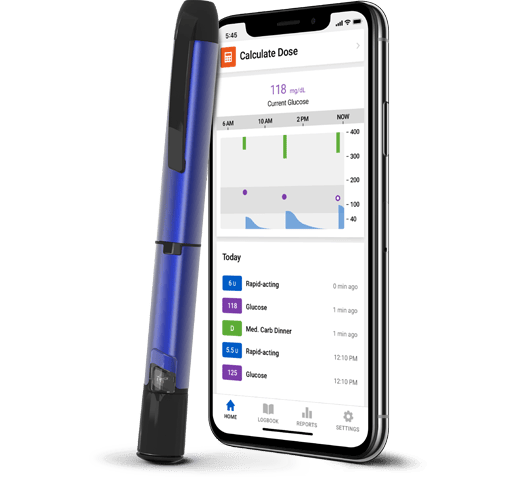
Smart MDI System
For people who prefer insulin injections
Products sold separately.
If you manage your diabetes with multiple daily injections (MDI), the smart insulin injection system can help reduce the physical and mental effort required to manage diabetes.
With a Smart MDI system, you get:
- Insulin tracking and dose reminders
- Dosing recommendations
- Actionable glucose alerts
Learn more
Understanding the basics

If you’d like some foundational understanding of what it may mean to sign up for Medicare, we recommend visiting the Medicare website or calling 1-800-MEDICARE (1-800-633-4227).
Ordering ongoing supplies

To make things easier, sign up for scheduled orders for ongoing shipments. We still need to connect with you before the shipment is sent.
If you prefer, you can place your order online at Diabetes.shop or by phone.
Speaking with a specialist

Speak to a Medtronic team member who can answer all your questions by calling 1-800-646-4633.
Existing customer? Use option 2 New to Medtronic? Use option 3
References
* Taking a bolus 15 – 20 minutes before a meal helps to keep blood sugar levels under control after eating.
** Fingersticks required in manual mode and to enter SmartGuard™. If symptoms don’t match alerts and readings, use a fingerstick. Refer to user guide. Pivotal trial participants spend avg of > 93% in SmartGuard™.
Important Safety Information: MiniMed™ 780G System With SmartGuard™ Technology With Guardian™ 4 Sensor
The MiniMed™ 780G system is intended for continuous delivery of basal insulin at selectable rates, and the administration of insulin boluses at selectable amounts for the management of type 1 diabetes mellitus in persons seven years of age and older requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 780G system includes SmartGuard™ technology, which can be programmed to automatically adjust insulin delivery based on the continuous glucose monitoring (CGM) sensor glucose values and can suspend delivery of insulin when the sensor glucose (SG) value falls below or is predicted to fall below predefined threshold values.
The Medtronic MiniMed™ 780G system consists of the following devices: MiniMed™ 780G insulin pump, the Guardian™ 4 transmitter, the Guardian™ 4 sensor, One-press serter, the Accu-Chek™ Guide Link blood glucose meter, and the Accu-Chek™ Guide test strips. The system requires a prescription from a healthcare professional.
The Guardian™ 4 sensor is intended for use with the MiniMed™ 780G system and the Guardian 4 transmitter to monitor glucose levels for the management of diabetes. The sensor is intended for single use and requires a prescription. The Guardian™ 4 sensor is indicated for up to seven days of continuous use.
The Guardian™ 4 sensor is not intended to be used directly to make therapy adjustments while the MiniMed™ 780G is operating in manual mode. All therapy adjustments in manual mode should be based on measurements obtained using a blood glucose meter and not on values provided by the Guardian™ 4 sensor. The Guardian™ 4 sensor has been studied and is approved for use in patients ages 7 years and older and in the arm insertion site only. Do not use the Guardian™ 4 sensor in the abdomen or other body sites including the buttocks, due to unknown or different performance that could result in hypoglycemia or hyperglycemia.
WARNING: Do not use the SmartGuard™ feature for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in the SmartGuard™ feature.
WARNING: Do not use the MiniMed™ 780G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 780G system.
WARNING: Do not use SG values to make treatment decisions, including delivering a bolus, while the pump is in Manual Mode. When the SmartGuard™ feature is active and you are no longer in Manual Mode, the pump uses an SG value, when available, to calculate a bolus amount. However, if your symptoms do not match the SG value, use a BG meter to confirm the SG value. Failure to confirm glucose levels when your symptoms do not match the SG value can result in the infusion of too much or too little insulin, which may cause hypoglycemia or hyperglycemia.
Pump therapy is not recommended for people whose vision or hearing does not allow for the recognition of pump signals, alerts, or alarms. The safety of the MiniMed™ 780G system has not been studied in pregnant women, persons with type 2 diabetes, or in persons using other anti-hyperglycemic therapies that do not include insulin. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult https://www.medtronicdiabetes.com/important-safety-information#minimed-780g and the appropriate user guide at https://www.medtronicdiabetes.com/download-library
Important Safety Information: InPen™
The InPen™ is a home- use reusable pen injector for single- patient use by people with diabetes under the supervision of an adult caregiver, or by a patient age 7 and older for the self- injection of a desired dose of insulin and for calculating an insulin dose or carbohydrate intake based on user entered data. A healthcare professional must assist in dosage programming of the device prior to use, based on various patient- specific criteria and targets. The InPen™ requires a prescription. For additional product and safety information, please consult the Instructions for Use and bit.ly/InPenRisks.

Ascensia, the Ascensia Diabetes Care logo, and Contour are trademarks and/or registered trademarks of Ascensia Diabetes Care.