What are automated insulin delivery systems?
Learn about how AID systems work and why they are the standard of care for type 1 diabetes.1
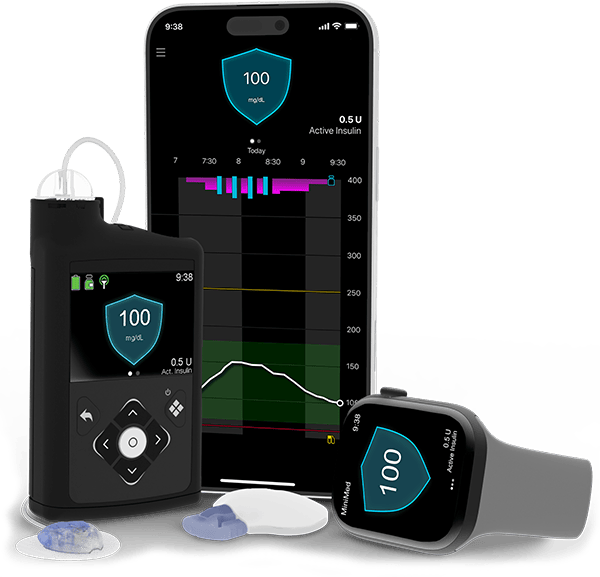
Automated insulin delivery (AID) systems
Automated insulin delivery (AID) systems combine an insulin pump and continuous glucose monitor (CGM) to help people living with type 1 diabetes manage their blood sugar levels.
AID systems, also known as hybrid closed loop systems, can detect changes in a person’s sugar levels in real time and automatically adjust insulin doses in response.
AID systems for kids are available depending on what insulin pump therapy your healthcare provider may recommend.How do AID systems work?
AID systems are made up of a CGM, an insulin pump, and a smart algorithm that links the two devices together, allowing them to “talk” to each other.
The CGM tracks your sugar levels every few minutes through a small sensor inserted under the skin.
Then the algorithm uses that data to tell your pump just the right amount of insulin to deliver. It may even tell the pump to stop sending insulin if your sugar levels are low.
While you still need to bolus before meals, the MiniMed™ 780G system is designed with SmartGuard™ technology that does the work in between. If your sugar levels are trending high, the system automatically gives you more insulin. If your sugar levels are trending low, the system automatically reduces the amount of insulin delivered.
Benefits of AID systems
Advanced technology helps make diabetes management easier
The smart algorithm that allows the CGM and insulin pump to “talk” to each other does so much more than just send sugar level data.
The algorithm uses current and past sugar level trends to anticipate, adjust, and correct insulin delivery.
AID systems, like the MiniMed 780G system, help take the guesswork out of counting carbs. It uses Meal Detection™ technology† to detect any miscalculated or occasionally missed meal doses and then deliver a correction dose.The standard in diabetes care
It’s no surprise why the American Diabetes Association calls automated insulin delivery systems the standard of care for people with type 1 diabetes. When your sugar levels are well-managed, it can help to:

The MiniMed 780G system
The only AID system with Meal Detection technology
Not all AID systems are created equal. Only the MiniMed 780G system with Meal Detection technology† can automatically deliver correction‡ boluses of insulin without any work needed from you, as quickly as every 5 minutes.
With the MiniMed 780G system, it works for you all day and night. In fact, 95 percent of customers felt more freedom from having to think so much about their diabetes.צ1
Learn about MiniMed 780G system
References
†Taking a bolus 15 – 20 minutes before a meal helps to keep blood sugar levels under control after eating.
‡ Refers to SmartGuard™ feature. Individual results may vary.
¶. Adults, T1 and parents of children with T1 diabetes < 18 years were surveyed.
◊. Individual results may vary.
1. dQ&A US Diabetes Patient Panel Report; Customer Overall Satisfaction, n=146; Q4 2023: P.52 (November 2023).
Important Safety Information: MiniMed™ 780G system with SmartGuard™ technology with Instinct sensor, Simplera Sync™ sensor, and Guardian™ 4 sensor
The MiniMed™ 780G system is intended for the continuous delivery of basal insulin at selectable rates and the administration of insulin boluses at selectable rates for the management of type 1 diabetes mellitus in persons 7 years of age and older, and of type 2 diabetes mellitus in persons 18 years of age and older requiring insulin. The system is also intended to continuously monitor glucose vales in the fluid under the skin.
The MiniMed™ 780G System includes SmartGuard™ technology, which can be programmed to automatically adjust insulin delivery based on the continuous glucose monitoring (CGM) sensor glucose values and can suspend delivery of insulin when the sensor glucose (SG) value falls below or is predicted to fall below predefined threshold values. The system is intended for use with connected sensors, including the Simplera Sync™ and Guardian™ 4 sensors and integrated continuous glucose monitors, including the Instinct sensor, each of which has different wear-time, form factor, insertion site, and other distinguishing characteristics that relate to sensor performance. Consult the appropriate sensor user guide when using the system. Discuss treatment decisions with your HCP.
WARNING: Do not use the SmartGuard™ feature for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in the SmartGuard™ feature.
WARNING: Do not use the MiniMed™ 780G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 780G system.
WARNING: Do not use SG values to make treatment decisions, including delivering a bolus, while the pump is in Manual Mode. When the SmartGuard™ feature is active and you are no longer in Manual Mode, the pump uses an SG value, when available, to calculate a bolus amount. However, if your symptoms do not match the SG value, use a blood glucose (BG) meter to confirm the SG value. Failure to confirm glucose levels when your symptoms do not match the SG value can result in the infusion of too much or too little insulin, which may cause hypoglycemia or hyperglycemia.
Pump therapy is not recommended for people whose vision or hearing does not allow for the recognition of pump signals, alerts, or alarms. The safety of the MiniMed™ 780G system has not been studied in pregnant women or in persons using other anti-hyperglycemic therapies that do not include insulin. For complete details of the system, including accessories and additional important safety information concerning indications, contraindications, warnings and precautions associated with the system and its components, please consult https://www.medtronicdiabetes.com/important-safety-information and the appropriate user guides at https://www.medtronicdiabetes.com/download-library.