Met your annual insurance deductible?
Now may be the best time to upgrade to the MiniMed™ 780G system with the Instinct sensor, made by Abbott, for an out-of-pocket cost as low as $0 if you have met your insurance deductible and coinsurance maximums.

Goodbye rollercoaster, hello stability
People who used the MiniMed™ 780G system benefited from automatic insulin adjustments and corrections every 5 minutes,† providing more consistency and fewer surprises.
Get more out of your diabetes management

The system adjusts insulin while you sleep, meaning fewer beeps and alarms.

The system requires as few as 6 device insertions a month with the 15-day Instinct sensor and 7-day Extended™ infusion set.§◊
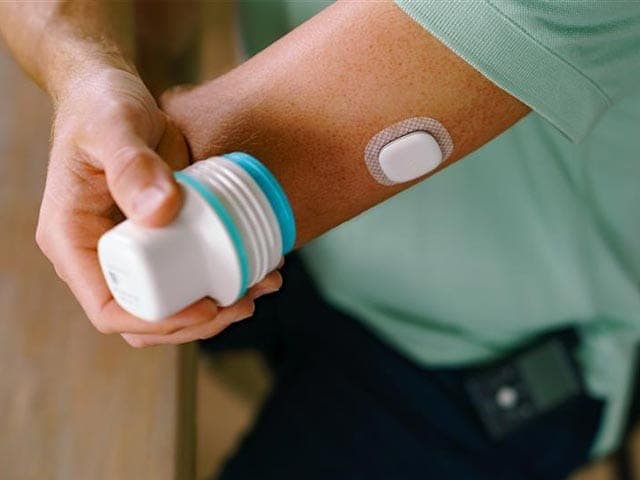
The system works with multiple sensors, including the new Instinct sensor, made by Abbott, and the Simplera Sync™ sensor. See options
Frequently asked questions
Medtronic insulin pumps are covered by most insurance plans. Your out-of-pocket cost varies depending on your insurance. We also offer financial assistance and monthly payment programs. If you would like to receive an insurance coverage check, call us at 1-888-350-5440 (M-F 9:00 a.m. – 6:00 p.m. CT).
For customers currently on a Medtronic insulin pump, sign in to Diabetes.shop to see your options.
The Simplera Sync sensor has coverage for Medicare, Medicare Advantage, and commercial insurance under your medical benefit. If your health plan requires you to use your pharmacy benefit, we will verify if the Simplera Sync sensor is covered by your plan.
The Instinct sensor has coverage for commercial insurance under your medical benefit. If your health plan requires you to use your pharmacy benefit, we will verify if the Instinct sensor is covered by your plan. If you have Medicare or Medicare Advantage, please note that we are currently waiting for billing code approval, which is expected in January 2026. In the meantime, you may place an order for the Instinct sensor and we'll process and ship your order at that time.
In a case of non-coverage, Medtronic offers programs to assist with access.
No. The only sensor made by Abbott that works with the MiniMed 780G system is the Instinct sensor.
If you currently use an in-warranty insulin pump from another company, you may be eligible for our Switch2System program when you trade in your current device.
Simply trade in your existing pump and pay an upgrade fee of just $499 to switch to the MiniMed™ 780G system. If it doesn’t work out, no problem! Just return it within 90 days. Learn more about our Switch2System program.
© Medtronic. MiniMed and MiniMed logo are trademarks of Medtronic MiniMed, Inc. The sensor shape and appearance, Abbott, and “a” logo are marks and/or designs of the Abbott group of companies in various territories and used under license. Sensor image © Abbott. ™*Third–party brands are trademarks of their respective owners.
Footnotes
† Refers to auto correct, which provides bolus assistance. Can deliver all correction doses automatically without user interaction, feature can be turned on and off.
‡ Due to inherent real-world study limitations, caution is advised when attempting to extrapolate these results to new patients. There could be significant differences. Overnight period: 3:00 AM- 6:00 AM. Number of users with ≥10 days of sensor glucose data; Optimal settings are glucose target at 100 mg/dL (5.5 mmol/L) and Active Insulin Time (AIT) at 2 hours and used >95% of the time.
§ Based on usage of 2 Instinct sensors for 15 days each and 4 Extended Infusion Sets for 7 days each in a one-month period. More insertions may be required if Instinct sensor or Extended infusion set requires replacement before specified wear time.
◊ Fingersticks required in manual mode & to enter SmartGuard™. If symptoms don’t match alerts & readings, use a fingerstick. Refer to user guide. Pivotal trial participants spend avg of > 93% in SmartGuard™.
1. McVean, et al. 972-P: Hyperglycemia from Real-World Dawn Phenomenon Is Nearly Eliminated with the MiniMed 780G System (MM780G). Diabetes 14 June 2024; 73 (Supplement_1): 972–P. https://doi.org/10.2337/db24-972-P
Important safety information: MiniMed™ 780G system with SmartGuard™ technology with Instinct™ sensor
The MiniMed™ 780G system is intended for continuous delivery of basal insulin at selectable rates, and the administration of insulin boluses at selectable amounts for the management of type 1 diabetes mellitus in persons seven years of age and older requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 780G System includes SmartGuard™ technology, which can be programmed to automatically adjust insulin delivery based on the continuous glucose monitoring (CGM) sensor glucose values and can suspend delivery of insulin when the sensor glucose (SG) value falls below or is predicted to fall below predefined threshold values.
The Instinct sensor can be used one time and has a life up to 15 days. The Instinct sensor is not intended to be used directly to make therapy adjustments while the MiniMed™ 780G system is operating in manual mode. All therapy adjustments in Manual mode should be based on measurements obtained using a blood glucose meter and not on values provided by the Instinct sensor. The Instinct sensor has been studied and is approved for use in patients ages 7 years and older and in the arm insertion site only. Only apply the sensor to the back of your upper arm. The sensor may not work properly in other areas.
The Medtronic MiniMed™ 780G System consists of the following devices: MiniMed™ 780G Insulin Pump and the Instinct sensor. The system requires a prescription from a healthcare professional.
WARNING: Do not use the SmartGuard™ feature for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in the SmartGuard™ feature.
WARNING: Do not use the MiniMed™ 780G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 780G system.
WARNING: Do not use SG values to make treatment decisions, including delivering a bolus, while the pump is in Manual Mode. When the SmartGuard™ feature is active and you are no longer in Manual Mode, the pump uses an SG value, when available, to calculate a bolus amount. However, if your symptoms do not match the SG value, use a blood glucose (BG) meter to confirm the SG value. Failure to confirm glucose levels when your symptoms do not match the SG value can result in the infusion of too much or too little insulin, which may cause hypoglycemia or hyperglycemia.
Pump therapy is not recommended for people whose vision or hearing does not allow for the recognition of pump signals, alerts, or alarms. The safety of the MiniMed™ 780G system has not been studied in pregnant women, persons with type 2 diabetes, or in persons using other anti-hyperglycemic therapies that do not include insulin. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult https://www.medtronicdiabetes.com/important-safety-information#minimed-780g and the appropriate user guide at https://www.medtronicdiabetes.com/download-library
Important safety information: MiniMed™ 780G system with SmartGuard™ technology with Simplera Sync™ sensorThe MiniMed™ 780G system is intended for the continuous delivery of basal insulin at selectable rates, and the administration of insulin boluses at selectable rates for the management of type 1 diabetes mellitus in persons 7 years of age and older, and of type 2 diabetes mellitus in persons 18 years of age and older requiring insulin. The system is also intended to continuously monitor glucose values in the fluid under the skin. The MiniMed™ 780G system includes SmartGuard™ technology, which can be programmed to automatically adjust insulin delivery based on continuous glucose monitoring (CGM) sensor glucose values and can suspend delivery of insulin when the SG value falls below or is predicted to fall below predefined threshold values.
The Simplera Sync™ sensor can be used one time and has a life up to 6 days, followed by a grace period of 24 hours. During the grace period, the sensor will continue to work as it did during the first 6 days, to allow the patient to change their sensor more flexibly. However, some sensors may not survive the full wear period for a variety of reasons. Please be prepared to replace the sensor during the grace period to ensure sensor glucose values continue to be monitored.
The Simplera Sync™ sensor is not intended to be used directly to make therapy adjustments while the MiniMed™ 780G is operating in manual mode. All therapy adjustments in Manual mode should be based on measurements obtained using a blood glucose meter and not on values provided by the Simplera Sync™ sensor. The Simplera Sync™ sensor has been studied and is approved for use in patients ages 7 years and older and in the arm insertion site only. Do not use the Simplera Sync™ sensor in the abdomen or other body sites, including the buttocks, due to unknown or different performance that could result in hypoglycemia or hyperglycemia.
The Medtronic MiniMed™ 780G System consists of the following devices: MiniMed™ 780G Insulin Pump, Simplera Sync™ sensor, the Accu-Chek™ Guide Link blood glucose meter, and the Accu-Chek™ Guide Test Strips. The system requires a prescription from a healthcare professional.
WARNING: Do not use the SmartGuard™ feature for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in the SmartGuard™ feature.
WARNING: Do not use the MiniMed™ 780G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 780G system.
WARNING: Do not use SG values to make treatment decisions, including delivering a bolus, while the pump is in Manual Mode. When the SmartGuard™ feature is active and you are no longer in Manual Mode, the pump uses an SG value, when available, to calculate a bolus amount. However, if your symptoms do not match the SG value, use a blood glucose (BG) meter to confirm the SG value. Failure to confirm glucose levels when your symptoms do not match the SG value can result in the infusion of too much or too little insulin, which may cause hypoglycemia or hyperglycemia.
Pump therapy is not recommended for people whose vision or hearing does not allow for the recognition of pump signals, alerts, or alarms. The safety of the MiniMed™ 780G system has not been studied in pregnant women, persons with type 2 diabetes, or in persons using other anti-hyperglycemic therapies that do not include insulin. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult https://www.medtronicdiabetes.com/important-safety-information#minimed-780g and the appropriate user guide at https://www.medtronicdiabetes.com/download-library
Important safety information: MiniMed™ 780G system with SmartGuard™ technology with Guardian™ 4 sensor
The MiniMed 780G system is intended for the continuous delivery of basal insulin at selectable rates, and the administration of insulin boluses at selectable amounts for the management of type 1 diabetes mellitus in persons 7 years of age and older, and of type 2 diabetes mellitus in persons 18 years of age and older requiring insulin. The system is also intended to continuously monitor glucose values in the fluid under the skin. The MiniMed 780G system includes SmartGuard technology, which can be programmed to automatically adjust insulin delivery based on continuous glucose monitoring (CGM) sensor glucose values and can suspend delivery of insulin when the SG value falls below or is predicted to fall below predefined threshold values.
The Medtronic MiniMed™ 780G system consists of the following devices: MiniMed™ 780G Insulin Pump, the Guardian™ 4 Transmitter, the Guardian™ 4 Sensor, One-press serter, the Accu-Chek™ Guide Link blood glucose meter, and the Accu-Chek™ Guide Test Strips. The system requires a prescription from a healthcare professional.
The Guardian™ 4 Sensor is intended for use with the MiniMed™ 780G system and the Guardian 4 transmitter to monitor glucose levels for the management of diabetes. The sensor is intended for single use and requires a prescription. The Guardian™ (4) sensor is indicated for up to seven days of continuous use.
The Guardian™ 4 sensor is not intended to be used directly to make therapy adjustments while the MiniMed™ 780G is operating in manual mode. All therapy adjustments in manual mode should be based on measurements obtained using a blood glucose meter and not on values provided by the Guardian™ 4 sensor. The Guardian™ 4 sensor has been studied and is approved for use in patients ages 7 years and older and in the arm insertion site only. Do not use the Guardian™ 4 sensor in the abdomen or other body sites including the buttocks, due to unknown or different performance that could result in hypoglycemia or hyperglycemia.
WARNING:Do not use the SmartGuard™ feature for peoplewho require lessthan 8 units or more than 250 units of total daily insulin per day. A total daily dose of atleast 8 units, but no more than 250 units, isrequired to operate in the SmartGuard™feature.
WARNING: Do not use the MiniMed™ 780G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 780G system.
WARNING: Do not use SG values to make treatment decisions, including delivering a bolus, while the pump is in Manual Mode. When the SmartGuard™ feature is active and you are no longer in Manual Mode, the pump uses an SG value, when available, to calculate a bolus amount. However, if your symptoms do not match the SG value, use a BG meter to confirm the SG value. Failure to confirm glucose levels when your symptoms do not match the SG value can result in the infusion of too much or too little insulin, which may cause hypoglycemia or hyperglycemia.
Pump therapy is not recommended for people whose vision or hearing does not allow for the recognition of pump signals, alerts, or alarms. The safety of the MiniMed™780G system has not been studied in pregnant women, persons with type 2 diabetes, or in persons using other anti-hyperglycemic therapies that do not include insulin. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associatedwith system and its components, please consult https://www.medtronicdiabetes.com/important-safety-information#minimed-780g and the appropriate user guide at https://www.medtronicdiabetes.com/download-library
