MiniMed Champion
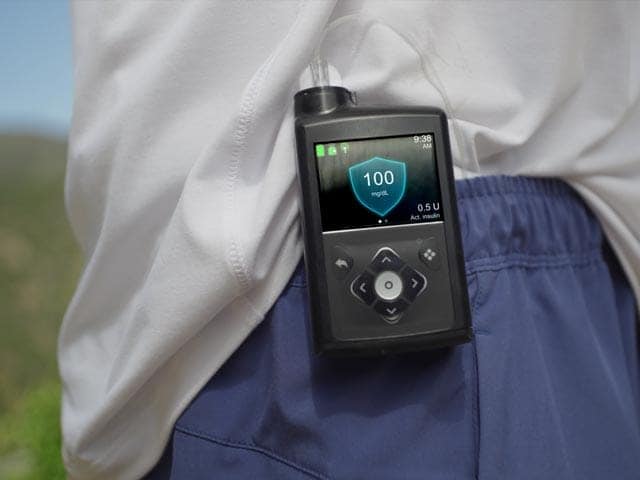
MiniMed™ 780G insulin pump system
Life with diabetes just got easier
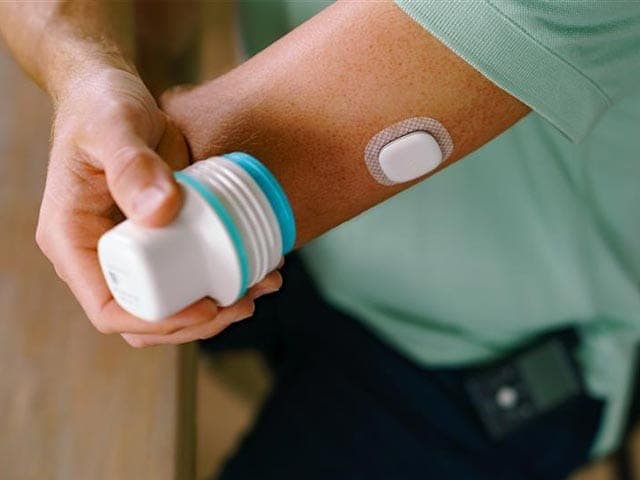
Experience the #1 pump in preventing highs and lows based on user satisfaction.1-3 Now even easier to use when paired with the new Instinct sensor, made by Abbott.

Small and discreet,4 the all-in-one sensor can be worn for up to 15 days.
Order nowGet more out of your diabetes management
Our system offers several features and benefits.

See how our insulin pump system works
- Anticipates — Predicts where your glucose levels are headed and how much insulin you’ll need based on your real-time data and past trends.
- Adjusts — Makes precise adjustments to insulin delivery to help keep you in range and protect from highs and lows.
- Corrects — Automatically corrects highs to help for undercounted carbs or a missed meal dose.¶
Meet the system
Order the MiniMed™ 780G system now and get our latest CGM technology.
Submit the form below and our team will reach out to discuss the option that’s right for you.
If you’re an existing customer, place your order on Diabetes.shop for quicker processing.
Still deciding?
Now you can try the MiniMed™ 780G system at no cost# with our Pump Evaluation Program.
Learn more
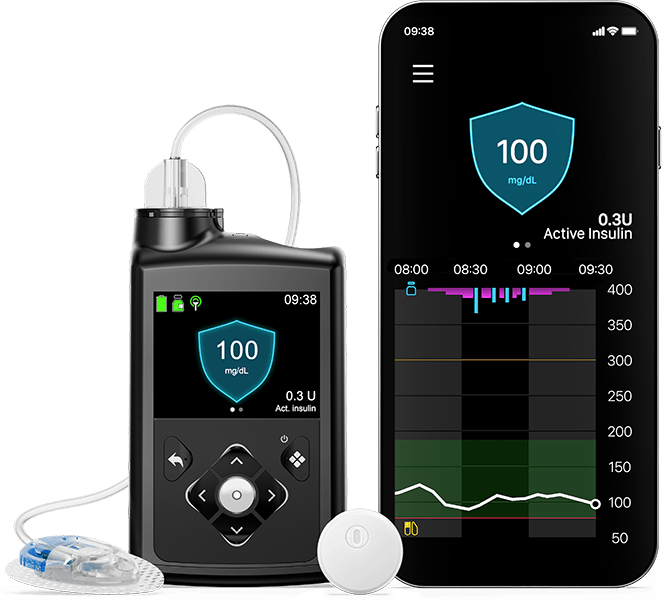
The MiniMed™ 780G system components
Get notified of highs and lows
Our mobile apps keep you in in the know!

See insulin pump data and glucose levels on your smart device.
Designed for care partners, remotely view the glucose levels and insulin pump information of a person with diabetes.
Reasons to choose an insulin pump
Benefits of insulin pump therapy include:
- Fewer injections — With an insulin pump, you insert an infusion set every few days. Multiple daily injections often require several shots each day. An insulin pump holds up to 300 units of insulin and delivers it discreetly with a few button pushes.
- Mealtime dosing — The bolus calculator eliminates complex math and tracks active insulin. Tracking active insulin can help avoid stacking and going low.
- More stable blood sugar — The system automatically adjusts basal insulin delivery all day and night to help you achieve better glucose control with fewer highs and lows.¶
- Individualized insulin delivery — An insulin pump delivers rapid-acting insulin and provides basal and bolus doses to better match your needs.
| Waterproof capabilities | At the time of manufacture and when the reservoir and tubing are properly inserted, your pump is waterproof. It is protected against the effects of being underwater to a depth of up to 12 feet (3.6 meters) for up to 24 hours. This is classified as IPX8 rating. See user guide for more details. The sensor and transmitter are water-resistant at 8 feet (2.4 meters) for up to 30 minutes. CGM readings may not be transmitted from the CGM to the pump while in water. The pump is not intended for submersion in water and is expected to be removed prior to swimming or bathing. |
|---|---|
| Environmental conditions | The MiniMed™ 780G insulin pump system is designed to withstand most conditions encountered in your daily life. Pump operating temperature range is from 41 °F (5 °C) to 98.6 °F (37 °C). Pump storage temperature range is from -4° F (-20° C) to 122° F (50° C). Air pressure range is from 700 hPa to 1060 hPa (10.2 psi to 15.4 psi). |
| Altitude range |
|
| Insulin type | Rapid-acting U100 insulin (Humalog® and NovoLog®) that has been prescribed by a healthcare professional. |
| Bolus delivery | Bolus Speeds
|
| Programming increments |
|
| Basal rate delivery | 0 to 35 units per hour or the Max Basal Rate amount, whichever is lower. |
| Screen |
|
| Battery & power | The pump requires one new AA (1.5 V) battery. For best results, use a new AA lithium (FR6) battery. The pump also accepts an AA alkaline (LR6) or a fully charged AA NiMH (HR6) nickel-metal hydride rechargeable battery. |
| Pump memory | At any time, you can review 30 days of pump history. |
| Size | The pump dimensions in inches are approximately 2.1 width x 3.78 length x 0.96 depth. The weight of the pump is approximately 3 ounces. |
| Warranty |
|
Compatible products |
|
| Reservoir | Medtronic reservoir MMT-332A, 3.0 ml (300-units), Medtronic reservoir MMT0326A, 1.8 ml (180-units). |
| Infusion sets | Medtronic Diabetes offers a wide range of infusion sets so that you can choose the right one for your comfort and safety. |
| Continuous glucose monitors (CGM) | Medtronic Diabetes offers a few options of CGM sensors so that you and your healthcare provider decide which is best for your needs and lifestyle. |
| Accu-Chek® Guide Link Blood Glucose Meter | The MiniMed™ 780G system comes with a compatible meter. It wirelessly connects to your pump, allowing you to send blood glucose meter readings to your pump. |
MiniMed insulin pumps are covered by most insurance plans. Your out-of-pocket cost varies depending on your insurance.
Medtronic offers financial assistance for qualifying patients and monthly payment programs. If you would like to receive an insurance coverage check, call us at 1-888-350-5440 (M-F 9:00 a.m. – 6:00 p.m. CT).
For customers currently on a MiniMed insulin pump, sign in to Diabetes.shop to see your options.
The Simplera Sync sensor has coverage for Medicare, Medicare Advantage, and commercial insurance under your medical benefit. If your health plan requires you to use your pharmacy benefit, we will verify if the Simplera Sync sensor is covered by your plan. If you don't have coverage, Medtronic offers programs to help.
The Instinct sensor has coverage for commercial insurance under your medical benefit. If your health plan requires you to use your pharmacy benefit, we will verify if the Instinct sensor is covered by your plan. If you have Medicare or Medicare Advantage, please note that we are currently waiting for billing code approval, which is expected in January 2026. In the meantime, you may place an order for the Instinct sensor and we'll process and ship your order at that time.
You can choose from any of our sensors options - Instinct, Simplera Sync or Guardian 4. Depending on the sensor you select, you may need to update your pump software but our latest software will work with all options. If so, we will let you know when you place your order.
You can place your order by going to Diabetes.shop. Our team will then work with your distributor to process your order.
No. The only sensor made by Abbott that works with the MiniMed 780G system is the Instinct sensor.
For adults, with the sensor worn in the back of the arm, the MARD is 8.2% overall.
The Instinct sensor will not accept calibrations, as the sensor is factory-calibrated. The MiniMed 780G system will be able to tell you if a blood glucose (BG) check is needed and guide you on next steps.
If your symptoms don’t match your sensor, check with your BG meter and enter the result. SmartGuard™ technology will adjust insulin delivery if the difference between your blood glucose (BG) value and your sensor glucose (SG) value is too great and guide you from there. SmartGuard™ technology is designed to operate safely, even if sensor performance varies, such as on the first day for a sensor.
If you currently use an in-warranty insulin pump from another manufacturer, you may be eligible for our Switch2System program when you trade in your current device.
Simply trade in your existing pump and pay an upgrade fee of just $499 to switch to the MiniMed™ 780G system. If it doesn’t work out, no problem! Just return it within 90 days. Learn more about our Switch2System program.
Adhering to your diabetes treatment plan and monitoring your glucose are important daily steps to take to prevent hyperglycemia concerns. However, the MiniMed™ 780G system has Meal Detection™ technology‡ that can detect if you’ve missed a meal dose and give you more insulin when you need it to help reduce post-meal hyperglycemia.† Always consult your healthcare team to find out what treatment is right for you.
To check if your smartphone or your Apple® Watch models are compatible, please visit our compatibility tool.
MiniMed™ Mobile app for people living with diabetes:
View your diabetes data on your smartphone or Apple® Watch, so you can easily track your glucose levels and get notified if you’re trending high or low. The app also enables automatic uploads of pump and CGM data to CareLink™ software. The MiniMed™ 780G system has the only diabetes mobile app from MiniMed compatible with select Apple® Watch. An iPhone® is required to enable the Apple® Watch extension.
If you are a healthcare professional, we encourage you to visit Medtronic professional diabetes healthcare website.
Footnotes
† Refers to auto correct, which provides bolus assistance. Can deliver all correction doses automatically without user interaction, feature can be turned on and off.
‡ Taking a bolus 15 – 20 minutes before a meal helps to keep blood sugar levels under control after eating.
§ Glucose targets should be chosen following a consultation with your healthcare provider.
◊ Due to inherent real-world study limitations, caution is advised when attempting to extrapolate these results to new patients. There could be significant differences. Overnight period: 3:00 AM- 6:00 AM. Number of users with ≥10 days of sensor glucose data; Optimal settings are glucose target at 100 mg/dL (5.5 mmol/L) and Active Insulin Time (AIT) at 2 hours and used >95% of the time.
¶ Refers to SmartGuard™ technology. Individual results may vary.
# Terms and conditions
References
1. Adults, T1 and parents of children with T1 diabetes < 18 years were surveyed
2. Individual results may vary.
3. dQ&A US Patient Voice Q2 2025.p. 77. N=215
4. Data on file, Abbott Diabetes Care, Inc.
5. McVean, et al. 972-P: Hyperglycemia from Real-World Dawn Phenomenon Is Nearly Eliminated with the MiniMed 780G System (MM780G). Diabetes 14 June 2024; 73 (Supplement_1): 972–P. https://doi.org/10.2337/db24-972-P
Program Terms and Conditions:
- Eligibility is limited to customers new to the MiniMed™ 780G System.
- A prescription is required.
- Participation is excluded for residents of Massachusetts, Michigan, Rhode Island and Minnesota.
- Participation is excluded for individuals enrolled in any federal, state or government-funded healthcare programs (such as Medicare, Medicaid, Tricare, etc.), Kaiser Permanente programs, or Excellus health programs.
- Evaluation items must be returned to Medtronic Diabetes within 90 days after completion of the evaluation period and cannot be sold or submitted for third[1]party reimbursement.
- Term and conditions are subject to change. Additional terms and conditions apply, see Patient Evaluation Program Patient Agreement for details.
Important Safety Information: MiniMed™ 780G system with SmartGuard™ technology with Instinct sensor, Simplera Sync™ sensor, and Guardian™ 4 sensor
The MiniMed™ 780G system is intended for the continuous delivery of basal insulin at selectable rates and the administration of insulin boluses at selectable rates for the management of type 1 diabetes mellitus in persons 7 years of age and older, and of type 2 diabetes mellitus in persons 18 years of age and older requiring insulin. The system is also intended to continuously monitor glucose vales in the fluid under the skin.
The MiniMed™ 780G System includes SmartGuard™ technology, which can be programmed to automatically adjust insulin delivery based on the continuous glucose monitoring (CGM) sensor glucose values and can suspend delivery of insulin when the sensor glucose (SG) value falls below or is predicted to fall below predefined threshold values. The system is intended for use with connected sensors, including the Simplera Sync™ and Guardian™ 4 sensors and integrated continuous glucose monitors, including the Instinct sensor, each of which has different wear-time, form factor, insertion site, and other distinguishing characteristics that relate to sensor performance. Consult the appropriate sensor user guide when using the system. Discuss treatment decisions with your HCP.
WARNING: Do not use the SmartGuard™ feature for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in the SmartGuard™ feature.
WARNING: Do not use the MiniMed™ 780G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 780G system.
WARNING: Do not use SG values to make treatment decisions, including delivering a bolus, while the pump is in Manual Mode. When the SmartGuard™ feature is active and you are no longer in Manual Mode, the pump uses an SG value, when available, to calculate a bolus amount. However, if your symptoms do not match the SG value, use a blood glucose (BG) meter to confirm the SG value. Failure to confirm glucose levels when your symptoms do not match the SG value can result in the infusion of too much or too little insulin, which may cause hypoglycemia or hyperglycemia.
Pump therapy is not recommended for people whose vision or hearing does not allow for the recognition of pump signals, alerts, or alarms. The safety of the MiniMed™ 780G system has not been studied in pregnant women or in persons using other anti-hyperglycemic therapies that do not include insulin. For complete details of the system, including accessories and additional important safety information concerning indications, contraindications, warnings and precautions associated with the system and its components, please consult https://www.medtronicdiabetes.com/important-safety-information and the appropriate user guides at https://www.medtronicdiabetes.com/download-library.
© Medtronic. MiniMed and MiniMed logo are trademarks of Medtronic MiniMed, Inc. The sensor shape and appearance, Abbott, and “a” logo are marks and/or designs of the Abbott group of companies in various territories and used under license. Sensor image © Abbott. ™*Third–party brands are trademarks of their respective owners.
DreaMed Diabetes is a trademark of DreaMed Diabetes, Ltd. The MiniMed™ 780G system algorithm includes technology developed by DreaMed Diabetes.