URGENT: MEDICAL DEVICE RECALL
Guardian™ 4 sensor accuracy issue
November 2023
Dear Valued Customer:
We’re reaching out because you may have received Guardian™ 4 sensors that have been identified to have a potential issue. Please carefully review the information below as it provides specific actions you should take.
Issue Description:
We’ve found that select Guardian™ 4 sensors in specific LOTs may potentially transmit inaccurate sensor glucose readings. We identified internally that the test system overseeing a portion of sensor assembly malfunctioned during the production of some sensor LOTs; this means some sensors may not have been tested properly and could potentially measure sensor glucose inaccurately. We’ve reviewed and updated our internal controls to avoid this issue going forward.
The MiniMed™ 780G system
If you are using an impacted Guardian™ 4 sensor with the MiniMed™ 780G system, an inaccurate sensor glucose reading may be used to determine if an auto correction or bolus is needed, which may result in over- or under-delivery of insulin, potentially resulting in hypoglycemia or hyperglycemia.
Actions:
To determine if you have sensors that are potentially impacted, please follow the steps below:
- Gather all your Guardian™ 4 sensors.
- Find the LOT number on the individual sensor box or sensor package.
Example of back of sensor box Example of sensor package Example of LOT number 

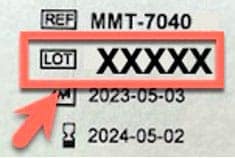
- Check your sensor LOT number(s) on our website at medtronicdiabetes.com/LOT, or in the list below to see if your sensors are impacted.
- If your LOT number(s) ARE NOT impacted, you can continue to use your sensors. IMPORTANT: Please read to the end of this letter for steps to acknowledge you have received and read this letter.
- If your LOT number(s) ARE impacted, do not use these sensors and request free replacement sensors by completing the web form at medtronicdiabetes.com/G4S-form, contacting 24-Hour Technical Support at 800-646-4633, option 1, or email customerexperience@medtronic.com
- If you cannot identify the LOT number of the sensor you are currently wearing, remove the sensor and replace your sensor with a non-impacted sensor.
- If you only have impacted sensors remaining, call us immediately so that we can ensure you receive replacement sensors as soon as possible. You should revert to your backup plan as recommended by your healthcare professional while you await your replacement sensors.
- Dispose of all impacted sensors.
Additional Information:
Please acknowledge that you have read and understood this notification and have followed the actions listed in this letter by completing and returning the attached confirmation form.
Adverse reactions or quality problems experienced with this product may also be reported to FDA’s MedWatch Adverse Event Reporting program:
- Submit online: http://www.fda.gov/medwatch/report.htm
- Submit by regular mail or fax: Download form http://www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
Your safety is our top priority, and we appreciate your time and attention in reading this important notification. We apologize for the inconvenience. If you have any questions, please contact us at 800-646-4633, option 1.
Sincerely,

Julio Salwen
Vice President, Quality
Medtronic Diabetes
If your LOT number is on this list, visit medtronicdiabetes.com/LOT or call 24- Hour Technical support at 800-646-4633, option 1 to request free replacement sensors. Please dispose of the impacted sensors after you request your replacements.
| Product Name | Product REF Number | Impacted LOT Numbers |
|---|---|---|
| Guardian™ 4 sensors | MMT-7040A | HG72BC, HG72CQQ, HG73VB7, HG73VC8, HG73VFT, HG73WFR, HG7441E, HG745F9, HG7486X, HG74AJ1, HG74SUR, HG74T9Q, HG74U36, HG78LMS, HG79Y9U |
| MMT-7040MA | HG74460 |
Frequently asked questions
Yes, by FDA definition, this is a recall. A “recall” as defined by the FDA, “does not always mean that you stop using the product or return it to the company. A recall sometimes means that the medical device needs to be checked, adjusted, or fixed.” (https://www.fda.gov/medical-devices/medical-device-recalls/what-medical-device-recall).
There is no retrieval activity required in this FCA; impacted customers are requested to check their products and if the product has a specific LOT number, stop using the product and request a replacement.
Your safety, awareness, and satisfaction are our top priorities. Keeping our customers informed when we find an issue is our top priority, and that is why we are notifying impacted customers of this issue.
Customers who have potentially impacted have been contacted through email or letter. The email or letter includes instructions for customers about how to identify if their sensors have been impacted.
To determine if you have an impacted sensor, follow the instructions in your email or letter. If your LOT number(s) ARE NOT on the list of impacted sensors, your sensors are not impacted, and you can continue to use your sensors. If your LOT number(s) ARE on the list of impacted sensors, do not use these sensors. Request a free replacement sensor by completing the webform or by contacting Medtronic 24-Hour Technical support at 1-800-646-4633, option 1.
Yes, Medtronic will provide replacements for all impacted sensors. Follow the instructions in your email or letter to determine if your sensors were impacted and to receive replacement sensors. However, if you are still having issues after the instructions, we are here to support you. Contact Medtronic 24-Hour Technical support at 1-800-646-4633, option 1.