Get the answers you need online.
Continuous glucose monitoring (CGM) with the MiniMed™ 670G system.

Top videos
Frequently asked questions
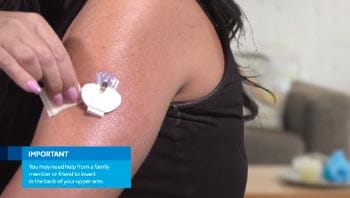
This system uses the Guardian™ Sensor 3. For those ages 14 and up, the sensor can be worn on the back of the upper arm or your abdomen. For those ages 7-13, the sensor can be worn on the abdomen or buttock.
We have a recommended method for taping that we have found is effective for most people who wear CGM. It requires you to use both pieces of oval tape that are supplied with your sensor. Some people also use an additional layer of tape called overtape. Watch the video below for step-by-step instructions on how to tape using this method.

- Many people have success wearing the sensor on the back of the upper arm.
- Follow our taping instructions! Incorrect taping is one of the most common reasons why sensors won’t stay in place.
- Try using a liquid adhesive to help your tape stick for longer. Make sure to apply the extra adhesive after you insert your sensor and before you put your tape on.
Watch the video below to see what other people who wear the system recommend.

You should calibrate when your glucose levels are stable. Exercise and eating can cause rapid changes in your glucose levels. That’s why many people choose to calibrate before meals and before bedtime. It’s important to always calibrate when the system asks you to or you will be exited from Auto Mode. For more information about calibrations, take a look at this video.

Alerts with the system are customizable. You can adjust your low, high, and calibration alert settings. Be sure to work with your healthcare provider who can help you determine how to optimize your settings.
For more information on how to adjust your low settings, click here.
For more information on changing your high settings, click here.
A few things might have happened.
- You might have received two consecutive “Calibration not accepted” notifications
- You accidently selected “No” on the “Check sensor insertion—Is sensor fully inserted?” screen
- The sensor might not be working properly and needs to be replaced
For all three scenarios, you will have to change your sensor.
Sometimes the transmitter can lose connection with the pump. This can be caused by a few things:
- The pump and transmitter are too far apart. If this happens, move the pump and transmitter closer together.
- There was interference between your pump and transmitter that interrupted the connection between the two devices. This can sometimes be caused by a cell phone or other electronic devices that emit radio frequency energy. If you experience this, try moving away from your electronic devices.
- Your sensor and transmitter may have become disconnected. The sensor itself can also contribute to potential losses in communication if it is pulled out from the skin. If this happens, you’ll need to insert a new sensor.
For more information about how to insert properly, click here.
To connect a transmitter to your pump, follow the steps below. If your pump already has a transmitter connected, you will need to delete that transmitter from the system prior to connecting a new one. If you need instructions on how to delete an old transmitter, click here.
- Place your transmitter on the charger and set it near your pump. Your transmitter must be fully charged to complete the following steps.
- Press Select.
- Select Options.
- Select Utilities.
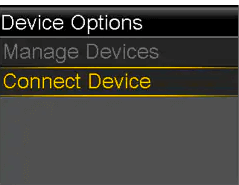
- Select Device Options.
- Select Connect Device.
- Select Auto Connect.
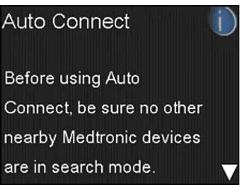
- Press the down arrow.
- Select Continue.
- Place the transmitter (still attached to the charger) next to the pump.
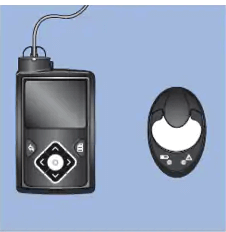
- Select Search on your pump and immediately remove the transmitter from the charger.

- Once the device is found, confirm that the serial number (SN) shown on the pump is the serial number on the back of your transmitter.

- If the serial number (SN) on the pump screen matches the back of the transmitter, select Confirm.
We recommend reordering sensors when you open your last box of 5 sensors. To order supplies, you have three options:
- Place your order at www.Diabetes.shop
- Text SUPPLY to 22094 to start the order process.
- Call us between 8am-6pm CST during the week at 800-646-4633, option 2.
If you order sensors through a distributor, you’ll need to contact them directly to place your next order.
Didn't find the answer you were looking for?
You can visit our system support page by clicking here.
If you bought your system within the last 3 months, you can also reach out to your onboarding support representative. Take a look at your welcome email with the subject line "How to reach us" for your representative's email address.
Important Safety Information: MiniMed™ 670G System with SmartGuard™ technology
The Medtronic MiniMed™ 670G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons, seven years of age and older, requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 670G system includes SmartGuard™ technology, which can be programmed to automatically adjust delivery of basal insulin based on Continuous Glucose Monitor sensor glucose values and can suspend delivery of insulin when the sensor glucose value falls below or is predicted to fall below predefined threshold values. The system requires a prescription. The Guardian™ Sensor (3) glucose values are not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. A confirmatory finger stick test via the CONTOUR®NEXT LINK 2.4 blood glucose meter is required prior to making adjustments to diabetes therapy. All therapy adjustments should be based on measurements obtained using the CONTOUR®NEXT LINK 2.4 blood glucose meter and not on values provided by the Guardian™ Sensor (3). Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the CONTOUR®NEXT LINK 2.4 blood glucose meter. Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an Alternative Site (palm) or from a control solution test. It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise. If a control solution test is out of range, please note that the result may be transmitted to your pump when in the “Always” send mode.
WARNING: Medtronic performed an evaluation of the MiniMed™ 670G system and determined that it may not be safe for use in children under the age of 7 because of the way that the system is designed and the daily insulin requirements. Therefore this device should not be used in anyone under the age of 7 years old. This device should also not be used in patients who require less than a total daily insulin dose of 8 units per day because the device requires a minimum of 8 units per day to operate safely.
Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. The safety of the MiniMed™ 670G system has not been studied in pregnant women. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult http://www.medtronicdiabetes.com/important-safety-information#minimed-670g and the appropriate user guide at http://www.medtronicdiabetes.com/download-library